This study investigates HLA-mismatched anti-CD19 CAR-NK cells derived from cord blood to patients with relapsed CD19-positive cancers (non-Hodgkin’s lymphoma or chronic lymphocytic leukemia .The results suggest that the majority of the patients had a response to treatment with CAR-NK cells without the development of major toxic effects.
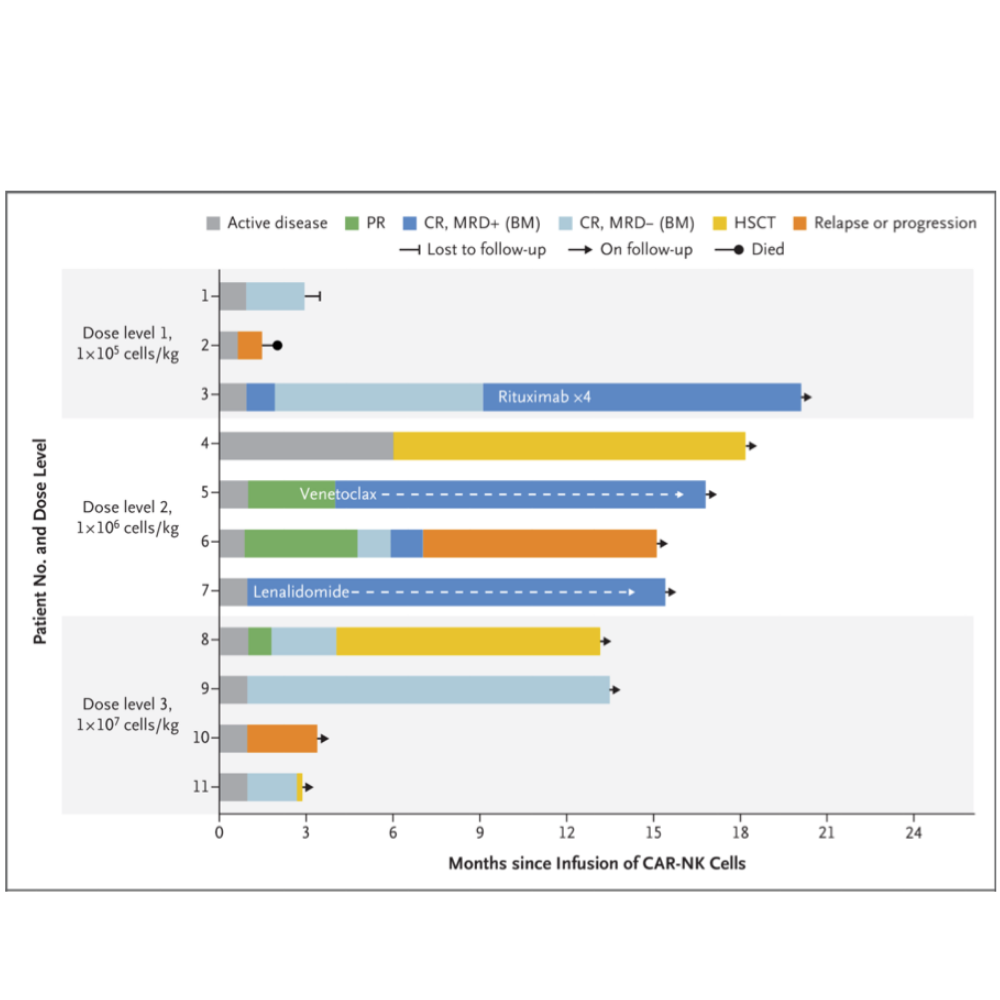
11 patients were enrolled in this study, five patients had CLL (including 2 who had Richter’s transformation or accelerated CLL), and all had a history of disease progression. Patients had lymphoma, including 2 with diffuse large B-cell lymphoma and 4 with the follicular form; 3 of these patients underwent transformation to high-grade lymphoma.
Manufacture of CAR-NK cells from Cord Blood
The cord-blood unit was thawed and NK cells were purified and cultured in the presence of engineered K562 feeder cells and interleukin-2. On day 6, cells were transduced with a retroviral vector encoding the genes for anti-CD19 CAR, the CD28.CD3ζ signaling endodomain, interleukin-15, and inducible caspase 9. The cells were expanded and harvested for fresh infusion on day 15. The efficiency of the final CAR-NK transduction for the infused product was 49.0% (range, 22.7 to 66.5). The median CD3-positive T-cell content in the infused product was 500 cells per kilogram (range, 30 to 8000), with a median of 0.01% (range, 0.01 to 0.002) contaminating CAR T cells in the product
Patients underwent lymphodepleting chemotherapy with fludarabine (at a dose of 30 mg per square meter of body-surface area) and cyclophosphamide (at a dose of 300 mg per square meter) daily for 3 consecutive days, followed by a single infusion of the trial CAR-NK cells at escalating doses of 1×105 cells, 1×106 cells, and 1×107 cells per kilogram of body weight.
Safety
After the infusion of CAR-NK cells, none of the patients had symptoms of cytokine release syndrome, neurotoxicity, or hemophagocytic lympho-histiocytosis. Moreover, there is no cases of graft-versus-host-disease, despite the HLA mismatch between the patients and their CAR-NK products.
All the patients had transient and reversible hematologic toxic events, which were mainly associated with the lymphodepleting chemotherapy. There were no cases of tumor lysis syndrome or grade 3 or 4 nonhematologic toxicity. The maximum tolerated dose of CAR-NK cells was notreached. No patient was admitted to an intensive care unit (ICU) for management of adverse events associated with CAR-NK cells.
Treatment response
At a median follow-up of 13.8 months (range 2.8 to 20.0), 8 patients (73%) had an objective response, including 7 patients (3 with CLL and 4 with lymphoma) who had a complete response.
An additional patient who had CLL with Richter’s transformation (Patient 5) had a complete remission of high-grade lymphoma, according to the absence of lesions with fluorodeoxyglucose uptake on positron-emission tomography– computed tomography (PET-CT) performed 30 days after the CAR-NK infusion.
These preliminary results show that CAR-NK cells can induce responses in patients with high-risk CD19-positive cancers with relatively few adverse events aside from transient myelotoxicity.
Article Reference link: click here
Scientific article publishing date: 8/6/2020
Article identifier BSC22_383EN
Más información

The foremost Cancer Therapy is here.