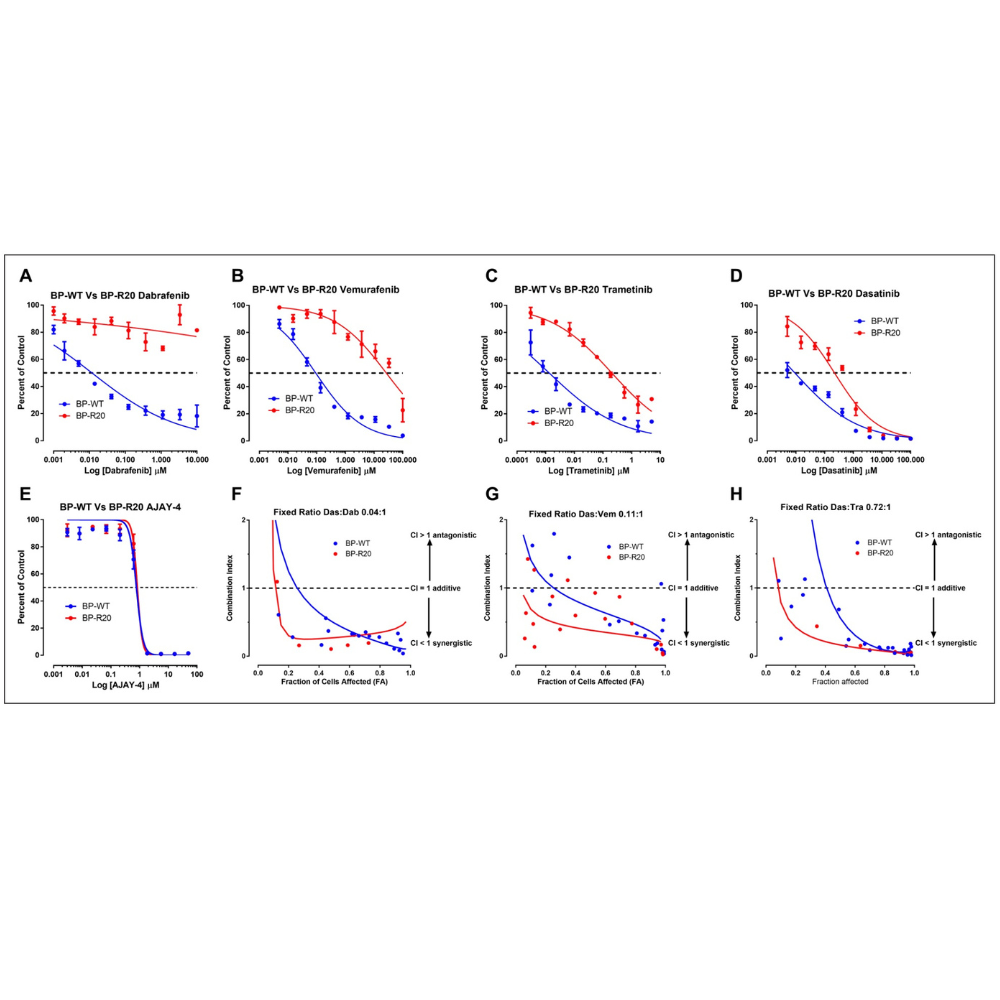
We describe the development, optimization, and validation of 384-well growth inhibition assays for six patient-derived melanoma cell lines (PDMCLs), three wild type (WT) for BRAF and three with V600E-BRAF mutations. We conducted a pilot drug combination (DC) high-throughput screening (HTS) of 45 pairwise 4×4 DC matrices prepared from 10 drugs in the PDMCL assays: two B-Raf inhibitors (BRAFi), a MEK inhibitor (MEKi), and a methylation agent approved for melanoma; cytotoxic topoisomerase II and DNA methyltransferase chemotherapies; and drugs targeting the base excision DNA repair enzyme APE1 (apurinic/apyrimidinic endonuclease-1/redox effector factor-1), SRC family tyrosine kinases, the heat shock protein 90 (HSP90) molecular chaperone, and histone deacetylases.
PDMCLs (Patient-Derived Melanoma Cell Lines) were established between 2007 and 2013 from four female and two male melanoma patients with tumors isolated from different anatomical sites. The cell lines were initiated after manual and enzymatic digestion of resected melanoma tumor samples from patients who had been subjected to a variety of different treatment regimens: individual or combination therapy with high-dose interferon-α, interleukin-2, melphalan perfusion, ipilimumab, or vemurafenib.
For the pilot DC HTS, a total of 45 pairwise 4×4 DCMs were generated from the 10 test compounds, and these were arrayed onto 3×384-well master plates. In addition to the nine DC wells, each 4×4 DCM contained a DMSO control and three single-drug control wells for each of the two drugs at concentrations tested within the matrix. Source A (40μL) and Source B (20μL) 10× master plates were arrayed manually using a matrix pipettor.
The 384-well transfer head of the Janus MDT Mini platform was used to transfer 20μL from Plate A into Plate B and mix the DC master plates. 20 μL of DMSO was added to single-drug wells, and 40 μL to DMSO control wells. The 384-well transfer head of the Janus MDT Mini was used to transfer 2 μL from the DC master plates into barcoded replica daughter plates, which were then centrifuged at 50×g for 5 min, sealed with aluminum foil, and stored at −20 °C until use.
These preclinical in vitro studies provide a data-driven rationale for the further investigation of DCs between dasatinib and BRAFis or MEKis as candidates for melanoma combination therapies with the potential to improve outcomes and/or prevent or delay the emergence of disease resistance.
Article Reference link: XX
Scientific article publishing date: 18/11/2020
Article Identifier BSC22_428EN
En savoir plus!

The foremost Cancer Therapy is here.
;