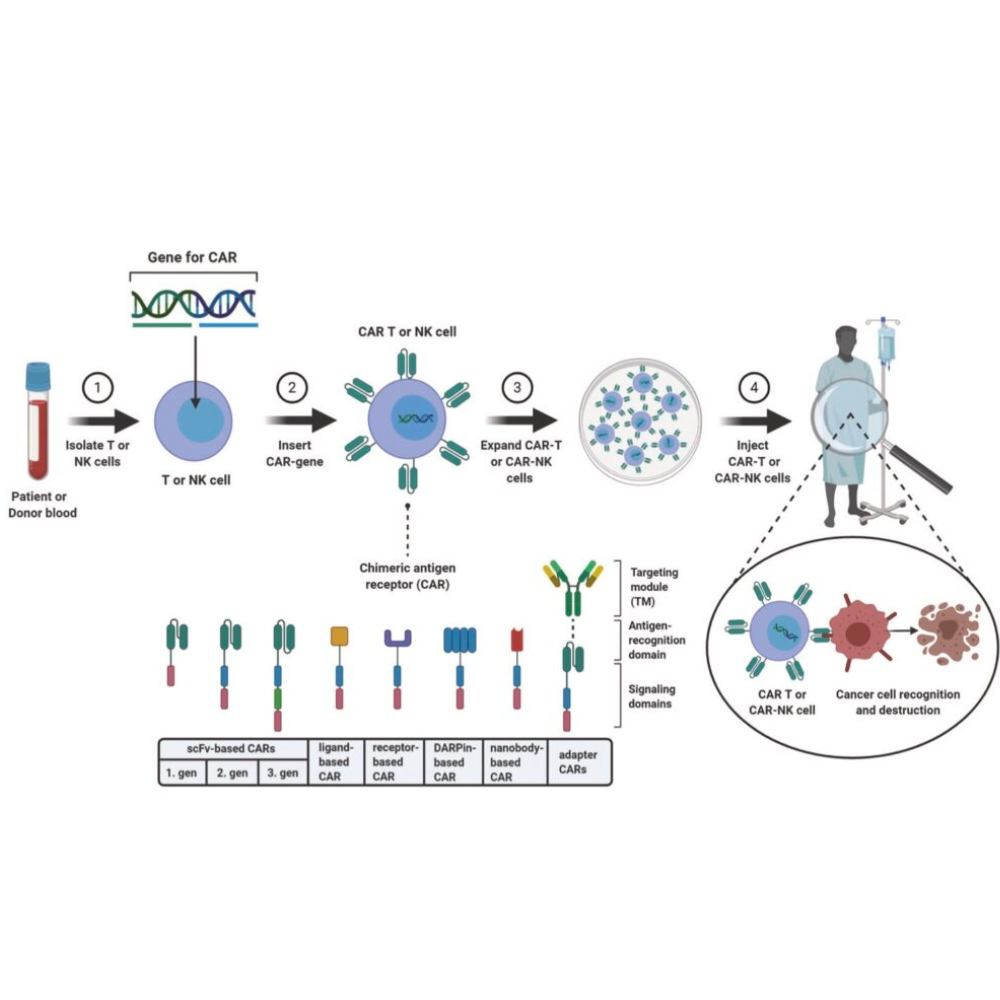
Recently, CAR-NK cell therapy has also come be the focus of new treatment options addressing barriers associated with CAR-T cell therapy, such as treatment-induced side effects.
Article Reference link: XX
Scientific article publishing date: 18/11/2020
Article Identifier BSC22_428EN

The foremost Cancer Therapy is here.
;
;