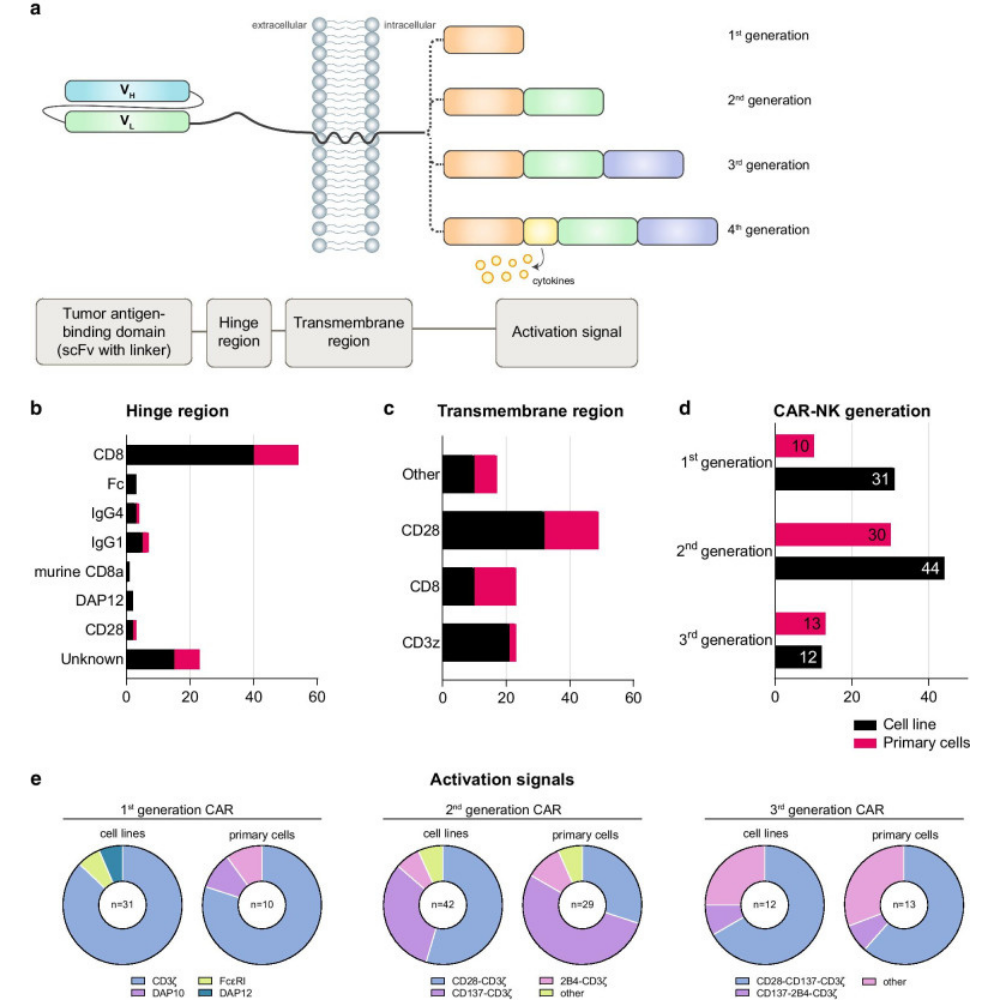
Natural killer (NK) cells are specialized immune cells that can be genetically manipulated to produce capable effector cells for adoptive cellular therapy of cancer patients due to their rapid detection and lysis of malignant cells. However, biological and technical barriers to gene delivery into NK cells have slowed advances significantly.
Various preclinical and a limited number of clinical studies using CAR-NK cells show promising results: efficient elimination of target cells without side effects, such as cytokine release syndrome and neurotoxicity which are seen in CAR-T therapie
Article Reference link: click here
Scientific article publishing date: 1/5/2021
Article identifier BSC22_412EN.

The foremost Cancer Therapy is here.
;
;